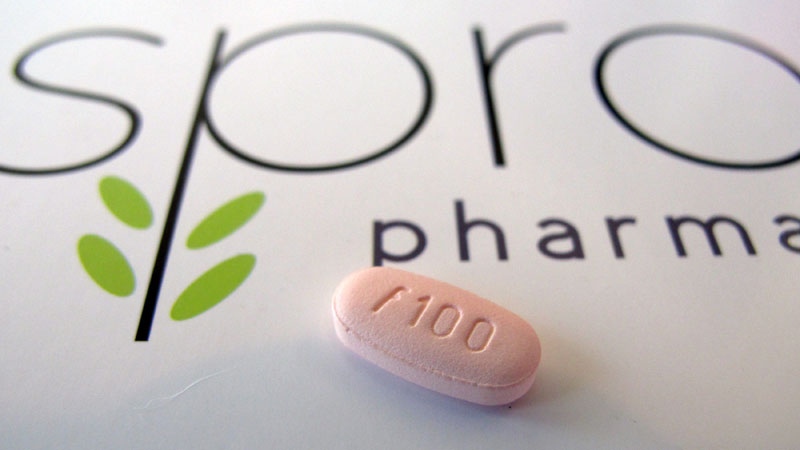
The makers of a controversial female libido pill that has just received approval from the U.S. Food and Drug Administration say they are now hoping to bring the drug to Canada.
Cindy Whitehead, the CEO of Sprout Pharmaceuticals, the small biotech company that developed the new drug, says they have already begun the process of seeking Health Canada’s approval for flibanserin, which is being marketed as Addyi.
“We’ve been in discussions but our intention is to file (for approval) in Canada in the fourth quarter of this year,” she told BNN on Thursday.
The FDA approved Addyi on Tuesday after rejecting the medication twice before, due to concerns about its effectiveness and significant side effects.
Sprout now plans to launch the drug in the U.S. in mid-October.
On Thursday, Canada's Valeant Pharmaceuticals International announced a friendly takeover of Sprout Pharmaceuticals so that the company could begin pursuing other international markets for the drug.
Addyi is designed to treat pre-menopausal women for a condition called “hypoactive sexual desire disorder,” otherwise known as low sex drive. Whitehead says research suggests one in 10 women suffer from the condition, which is described as a lack of sexual appetite that causes emotional distress in patients who previously had no problems with sexual desire.
Even the condition itself is controversial. Many psychologists say there are often many factors that affect sexual appetite, including relationship issues, depression, stress and mood disorders.
But many have also argued that women have been left out of treatments for sexual disorders, even while drugs to treat sexual dysfunction in men have received plenty of attention.
Those medications, which include Viagra, are designed to increase blood flow to the genitals and are meant to be taken shortly before intercourse. Addyi, on the other hand, acts on serotonin, a brain chemical that affects mood. In fact, the pill was originally developed as a treatment for depression before being repurposed into a drug for low libido.
Addyi also differs from drugs such as Viagra because it needs to be taken daily for several weeks before it becomes effective.
The drug maker’s own clinical trials have found the pill to be only modestly effective. Women in clinical studies reported on average one extra “sexually satisfying event” per month while using the medication. As well, about 10 per cent more Addyi-treated patients reported improvements in sexual desire compared to those taking a placebo.
The FDA noted in its approval that the medication carries the risk of serious side effects, including sudden fainting, and that women taking the drug need to be informed they cannot drink alcohol.
In the U.S., the drug's label will carry a boxed warning -- the most serious type of warning -- alerting patients that mixing alcohol with the medication can cause dangerously low blood pressure and fainting. That risk can also occur when taking the drug with other medications, such as those used to treat yeast infections. Other side effects include nausea, fatigue, insomnia and dry mouth.
The FDA is concerned enough about the risks that they are requiring doctors to complete an online certification test to demonstrate they understand Addyi’s side effects before they can prescribe it. They will also have to assess the likelihood that each patient can reliably abstain from alcohol while taking Addyi.
Sprout’s Whitehead says they are confident they have now taken all the steps needed to ensure the drug’s safety.
“We’ve done a lot of additional work to further inform the label and I think we have a label now that informs women about the benefits and the risks,” she told BNN.
She added there is an “extraordinary unmet need” for the medication and that her company is glad that women will now have a new option for treating sexual dysfunction.
“We’ve always been about making it to the finish line (with FDA approval) and letting women make the choice with their health care provider about this treatment,” she said.
With files from The Associated Press